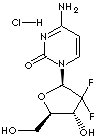
PRODUCT IDENTIFICATION
H.S. CODE
TOXICITY
CLASSIFICATION
PHYSICAL AND CHEMICAL PROPERTIES
286 - 292 C
SOLVENT SOLUBILITY
REFRACTIVE INDEX
Stable under ordinary conditions.
GENERAL DESCRIPTION & APPLICATIONS
gemcitabine hydrochloride: The hydrochloride salt of an analogue of the antimetabolite nucleoside deoxycytidine with antineoplastic activity. Gemcitabine is converted intracellularly to the active metabolites difluorodeoxycytidine di- and triphosphate (dFdCDP, dFdCTP). dFdCDP inhibits ribonucleotide reductase, thereby decreasing the deoxynucleotide pool available for DNA synthesis; dFdCTP is incorporated into DNA, resulting in DNA strand termination and apoptosis...... (source: http://www.cancer.gov)
Objective: To evaluate the cost effectiveness of gemcitabine in the treatment of nonsmall cell lung cancer (NSCLC). Methods: Gemcitabine was compared with best supportive care and gemcitabine/cisplatin was compared with three standard chemotherapies and four other novel chemotherapy combinations. Costs and effectiveness measures were based on resource and outcome data from previously reported clinical trials. All direct costs associated with NSCLC treatment were included and adjusted to year 2000 values. Perspective: UK National Health Service. Results: Gemcitabine plus best supportive care was associated with an incremental cost per progression-free life year gained of Lstg 5228 compared with best supportive care alone. In comparison with standard chemotherapies, gemcitabine/cisplatin was associated with an incremental cost per progression-free life year gained of Lstg 1751 versus etoposide/cisplatin and cost per 1-year survival gain of Lstg 5681 versus mitomycin/vinblastine/platinum. Incremental cost per tumour response was Lstg 2032 relative to etoposide/cisplatin, Lstg 5169 relative to mitomycin/ifosfamide/cisplatin and Lstg 6240 relative to mitomycin/vinblastine/platinum. Compared with four novel (newer) combination chemotherapies gemcitabine/ cisplatin showed cost savings in each case, with the same or better outcome. Thus, gemcitabine/cisplatin showed improved cost effectiveness and dominance. Sensitivity analyses showed the results were robust to variations to the values of key parameters. Conclusion: Gemcitabine alone or in combination with cisplatin was assessed to be a cost-effective or cost-saving therapy when compared with best supportive care, standard chemotherapy regimens and novel chemotherapy combinations. Chemotherapy regimens containing gemcitabine therefore represent good value for money and efficient use of healthcare resources in the treatment of advanced NSCLC........ (source: http://ideas.repec.org/)Gemcitabine inhibits thymidylate synthetase, leading to inhibition of DNA synthesis and cell death. Gemcitabine is a prodrug so activity occurs as a result of intracellular conversion to two active metabolites, gemcitabine diphosphate and gemcitabine triphosphate by deoxycitidine kinase. Gemcitabine diphosphate inhibits ribonucleotide reductase, the enzyme responsible for catalyzing synthesis of deoxynucleoside triphosphates required for DNA synthesis. Gemcitabine triphosphate (diflurorodeoxycytidine triphosphate) competes with endogenous deoxynucleoside triphosphates for incorporation into DNA........(source: http://www.mongabay.com/)
APPEARANCE
IDENTIFICATION
pass
ASSAY
98.0 - 102.0%
MELTING POINT
286 - 292 C
SPECIFIC ROTATION
RELATED SUBSTANCES
1.0% max